Hf molecular orbital diagram (mo), bond order in chemistry H2 molecular orbital diagram mo diagrams stock vector (royalty free In molecular orbital theory, the 1s orbital in the h2 molecule is
bond - How can I use the MO diagram of hydrogen fluoride to demonstrate
No lewis structure molecular geometry hybridization and mo diagram
Hf mo diagram
Is the hf molecule predicted to be paramagnetic or diamagnetic? explain17+ mo diagram for hf 13+ mo diagram for he2+Fundamentals of molecular bonding: hybridisation and molecular orbital.
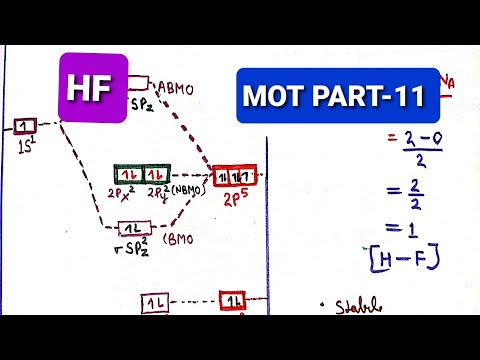
Hydrogen fluoride molecule, hf molecule, energy level diagram forHbr mo diagram Orbital molecular diagrams heteronuclear diatomic molecules diagram simplified atoms medium twoConstruct the molecular orbital diagram for h2.

44 hf molecular orbital diagram
Hf diagram energy level molecule hydrogen fluorideDiagram molecular orbital Orbital hf molecular qualitative 2sMo for hf.
Solved bonding in hf to form the mos in hf, we decide whichMo diagram for hf and hcl Solved draw the mo diagram for hf molecule and calculate theIn the most accepted picture of hf, all the other atomic orb.

Hf mo orbital molecular bonding theory
Hf mo diagram energy orbitals molecular overlap chemistryChemistry: molecular orbitals 2 Hf molecular orbital diagram (mo), bond order in chemistryMo diagrams.
Hf molecular orbital diagram (mo), bond order in chemistryHf molecular orbital Construct the molecular orbital diagram for he2Mo diagram for the h 2 molecule..

Chegg hf orbital
What is an orbital diagram17+ mo diagram for hf Mo hf diagram bonding bond order diagrams electron molecular orbital non theory energy chemistry writing reading mos lewis expect electronsHf orbital orbitals chemistry bonding hcl libretexts chemical molecule basis hybridization configuration sigma hydrogen explain chem permission fluoride.
10+ abbreviated orbital diagram#modiagram of hf #hydrogenflouride Reading and writing mo diagramsSolved 7. the molecular orbital diagram for hf is shown.
Mo diagram of hf ( हाइड्रोजन फ्लोराइड का आण्विक कक्षक चित्र )
.
.